Chemistry 2022
Objective Questions
The by-product of fermentation of sugar is
Correct Answer: A) Carbon (IV) oxide
Which of the following sugars is a product of the condensation of monosaccharides?
Correct Answer: B) Maltose
The cleansing effect of soap is low in acidic water because of
Correct Answer: A) The formation of unionized fatty acid
The following compounds are condensation polymers except
Correct Answer: D) Polyethene
What amount of electricity is required to deposit one mole of aluminium from a solution of AlCl₃?
Correct Answer: C) Three faradays
Which of the following compounds would react rapidly with bromine?
Correct Answer: C) Hexene
Alkanols can be manufactured from alkenes by the initial reaction of alkenes with
Correct Answer: B) Concentrated tetraoxosulphate (VI) acid
Which of the following statements about the standard hydrogen electrode is not correct?
Correct Answer: D) The temperature is kept at 20 °C
If 60 g of M combines with 24 g of oxygen,
what would the empirical formula of the oxide be?
(Molar
mass of M = 120, O = 16)
Correct Answer: D) MO₄
The products of the electrolysis of dilute sodium chloride using carbon electrodes are
Correct Answer: C) Chlorine and hydrogen
Determine the quantity of electricity used when a current of 0.20 amperes is passed through an electrolytic cell for 60 minutes.
Correct Answer: D) 720 C
Oxochlorate(I) acid is used as a bleaching agent because it is
Correct Answer: C) An oxidizing agent
The IUPAC name for CH₃CH(CH₃)CHClCH(CH₃)CH₂CH₃ is
Correct Answer: D) 3-chloro-2,4-dimethylhexane
A colourless gas with a pungent smell is evolved when dilute hydrochloric acid is added to a sample of a salt. The gas evolved could turn
Correct Answer: D) Pb(NO₃)₂ paper black
If 5.0 g of marble reacts with 25.0 cm³ hydrochloric acid, which of the following combinations has the fastest reaction rate?
Correct Answer: C) Powdered marble and 2.5 mol dm⁻³ HCl(aq)
Increasing the temperature generally
Correct Answer: B) Increases the solubility of a solid in a liquid but decreases the solubility of a gas in a liquid
A white precipitate was formed when BaCl₂(aq) was added to an aqueous solution of a salt X. The precipitate dissolved in dilute HCl with rapid effervescence. Salt X is likely to contain
Correct Answer: D) CO₃²⁻ ions
Before a reaction could take place, there should be
Correct Answer: C) Breakage of bonds of reactants
Consider the following reaction
equation:
CaO + SiO₂ → CaSiO₃
Silicon(IV) oxide is
acting as
Correct Answer: C) An acidic oxide
Which of the following acids would form normal salt only?
Correct Answer: C) Trioxonitrate(V) acid
What is the partial pressure of oxygen at s.t.p. in a gaseous mixture containing 100 cm³ of oxygen and 900 cm³ of nitrogen gas?
Correct Answer: B) 0.1 atm
Graphite is used as a dry lubricant due to the presence of
Correct Answer: D) Layered structures
Which of the following gases is alkaline?
Correct Answer: C) NH₃
Which of the following statements about an equilibrium system is correct?
Correct Answer: A) Forward and backward reactions occur at the same rate
Consider the following reaction
equation:
N₂(g) + 3H₂(g) ⇌ 2NH₃(g), ΔH = -92
kJ
Increasing the temperature of the reaction would
Correct Answer: D) Decrease the yield of ammonia
When air in a syringe is compressed such that there is no change in temperature, the
Correct Answer: B) Pressure increases
Which of the following statements about liquids is/are true?
I. Liquids maintain their volume at constant
temperature.
II. Liquids have fixed shape
III. Liquids
do not diffuse
IV. Change in pressure affects volume of
liquids
Correct Answer: A) I only
A hydrogen chloride gas reacted with oxygen gas to yield water and chlorine gas. The mole ratio of the hydrogen chloride gas to water is
Correct Answer: B) 2:1
What number of moles of oxygen would exert a
pressure of 10 atm at 320 K in an 8.2 dm³ cylinder?
[R =
0.082 atm·dm³·mol⁻¹·K⁻¹]
Correct Answer: C) 3.13
If 50 cm³ of a saturated solution of KNO₃ at 40°C contained 5.05 g of the salt, its solubility at the same temperature would be [KNO₃ = 101]
Correct Answer: A) 1.0 mol·dm⁻³
Which of the following elements would displace copper from a solution of copper ions?
Correct Answer: C) Lead
What is the percentage composition of carbon in Ca(HCO₃)₂? [Ca = 40.0, O = 16.0, C = 12.0, H = 1.0]
Correct Answer: B) 14.8%
Which of the following bond types is intermolecular?
Correct Answer: B) Hydrogen bond
The maximum number of covalent bonds formed by nitrogen is
Correct Answer: C) 3
The IUPAC name of the compound CH₃CH(CH₃)CH=CH₂ is
Correct Answer: C) 3-methylbut-1-ene
Ionization energy increases across the period in the periodic table because
Correct Answer: B) Effective nuclear charge increases
Which of the following properties indicate that an element is a metal? It:
I. Reacts with oxygen to form an acidic oxide
II. Forms
ionic chlorides
III. Has variable oxidation states
IV.
Displaces hydrogen from dilute HCl
Correct Answer: C) II and IV only
The electron configuration of a carbon atom in its excited state is:
Correct Answer: C) 1s² 2s¹ 2px¹ 2py¹ 2pz¹
An oxide has the following
properties:
I. It is a white powder
II. It reacts with HCl
III. It reacts with NaOH
IV. It is insoluble in water
The oxide is:
Correct Answer: B) Amphoteric
Which of the following statements about atoms of a metal is correct? They:
Correct Answer: D) Are held together by a sea of electron cloud
Which of the following sketches is a graphical representation of Boyle's law?

Correct Answer: C) C
The atom with the electron configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁴ is
Correct Answer: A) Period 4, p-block
Electropositivity of elements across the periodic table normally
Correct Answer: C) Decreases across the period
Consider the following table. Which of the substances is a liquid at room temperature and rapidly evaporates on exposure to air?
| Substitute | Meltig point / ºC | Boiling point / ºC |
| P | - 78 | -25 |
| Q | - 8 | 40 |
| R | - 6 | 150 |
| S | 44 | 280 |
Correct Answer: B) Q
Which of the following oxides is amphoteric?
Correct Answer: C) Lead(II) oxide
Which of the following processes occur during fractional distillation of petroleum?
Correct Answer: D) Evaporation and condensation
If the molar mass of X(HCO₃)₂ is 162 g mol⁻¹, determine the relative atomic mass of X. [H = 1.0, C = 12.0, O = 16.0]
Correct Answer: D) 101
Charcoal is used in the decolourization of sugar because of its
Correct Answer: A) Absorption property
The first definition of an element was made by
Correct Answer: C) R. Boyle
Which of the following scientists formulated the law of conservation of mass?
Correct Answer: A) A. Lavoisier
Theory Questions
(a)
i. Define an acid according to the Lewis concept
ii. Give one example of a Lewis acid
(b) Explain salting out in soap preparation
(c) State the reagent and condition necessary for the following
conversion
H−C≡C−H→Ag−C≡C−Ag
(d) What is the percentage abundance of an isotope?
(e)
i. Why does the element with atomic number 18 not have an
oxide?
ii. Explain why chlorine(I) oxide has a low melting point
(f). Describe a test to distinguish between concentrated
HNO3 and concentrated H2SO4
(g) State two differences between an electrochemical cell and an
electrolytic cell
(h) How does the trend in ionization energy affect the
reactivity of group 1 elements?
(i).Define the term molecular formula
(j) State which of the gases H2 and NH3
would deviate more from ideal behaviour. Give reasons for the
answer stated above
(a)
(i) Define the first ionization energy of an element
(ii) Consider the following table and use it to answer te
question that follows
| Element | Li | Be | b | C | N | O | F | Ne |
| Atomic number | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 1st I.E/kj mol-1 | 520 | 900 | 801 | 1086 | 1402 | 1314 | 1681 | 2081 |
Explain briefly why the first ionization energy of B is less than
that of Be despite the fact that the atomic number of B is
greater than that of Be.
(b) When Titanium chloride was electrolysed by passing 0.12 A
current through the solution for 500 seconds, 0.015 g of
titanium was deposited. What is the charge on the titanium
ion?
[ IF= 96500 C, Ti= 48.0 ]
(c)
(i) Aluminium can be obtained by the application of
electrolysis. State the electrolyte which yields aluminium on
electrolysis.
(ii) Name two major factors which would favour the siting of an
aluminium smelter in a country.
(d)(i) Define the term paramagnetism.
(ii) Consider the following ions: 24Cr2+
, 24Cr6+
(I) Deduce the number of unpaired electrons in each of the ions.
(II) State which of the ions will have a greater power of
paramagnetism
(l) Give a reason for the answer stated in (d)(ii)(II)
(a)
(i) Define the term Avogadro's number.
(ii) If 2.30 g of an oxide of nitrogen, x, contains 3.01 x 1022
molecules, calculate the molar mass of x.
(iii) Deduce the formula of x.
[NA, =6.02 x
1023, N =14.0, O = 16.0]
(b)
(i) Describe briefly what happens when each of the following
substances are added to water:
(I) CCI4
(II) SiCI4
(ii) Explain briefly why the reactions in (a)(i), (b)(i), (I)
and (b)(ii) (II) are different
Study the diagram below and
answer the questions that follow.
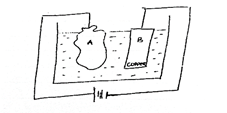
- What is the set-up used for?
- Mention two compound that could be used as electrolytes in the cell.
- Write a half-cell equation for the reaction at the anode.
- Calculate the electrochemical equivalent of copper if the cathode gained mass of 3.2 g when 50 amperes of current was passed for 5 mins 13 seconds.
(a)
i. State two conditions used in the Haber process
ii. Explain briefly the effect of increasing the pressure on
the rate of reaction in the Haber process.
(b)i. A mixture of nitrogen(IV) oxide and oxygen is bubbled
into warm water to produce
trioxonitrate(V) oxide, write a balanced chemical equation
for the reaction.
ii. Using a balanced chemical equation only, explain what
would happen if nitrogen(IV) oxide is bubbled into warm
water.
iii. Compare the gases evolved when trioxonitrate(V) acid
decomposes under each of the following properties:
i. pH
ii. Solubility in water
iii. Reaction with carbon(II) oxide
(c)
i. Name two oxides of sulphur
ii. Write a balanced equation for the reaction between each
of the named oxides(Sulphur(IV) oxide, Sulphur (VI) oxide)
and water
(d)
i. Name one calcium compound
a. Used to dry ammonia gas
b. Used in the manufacture of cement
c. That causes hardness in water
d. Name one calcium compound
(ii) Write a balanced equation for the reaction between each of the named oxides in 4(c)(i) and water.
(a) Describe the observation that would be made when
i. Sulphur is heated from room temperature till 1190C
ii. 50% trioxonitrate(V) acid acts on copper tunings
(b)
i. State two gaseous pollutants that can be generated
by burning coal.
ii. What gas is responsible for most of the explosions in
coal mines?
iii. The mining of coal leads to environmental pollution.
State two environmental effects of the mining activity.
iv. Explain briefly why coal burns more easily when it is in
pieces than in lump form
v. Name the non-volitile residue after the destructive
distillation of coal
(c)
i. Describe a chemical test for water
ii.
a. State the effect of boiling a temporary hard
water
b. State the effect of adding sodium trioxocarbonate(IV)
crystals to permanent hard water
iii. Write an equation for the process of boiling a
temporary hard water